Tuesday 24 August 2010
Evaporative cooling due to chemical reactions?
Monday 23 August 2010
Smart material control valves
Tancio suggested we could somehow incorporate smart materials into our control system for the adsorption refrigeration cycle.
Smart materials have special properties which cause them to undergo some specific change when induced to external stimuli.
Referring back to the idea of the termite ventilation system (where natural buoyancy forces are cleverly manipulated by dynamically opening/closing ducts to control the flow of air through the system at the correct rates to maintain the required temperatures.
Designing such a system would firstly require a thorough understanding of the thermodynamic principles of this complex system which may require computer modelling.
In any case, we would require some form of actuator to open and close the ducts. Since it may be difficult to hire an army of termites to do this for us, in our case we may be able to use smart materials our actuators.
Dielectric Elastomers (DE) and Shape Memory Alloys (SMA) are particular smart materials which may be useful for this application as they expand when induced to an electric current and heat respectively. They could therefore be used as air flow control valves by using their expansion process to constrict or open ducts as required.
If we need a valve which opens with an increase in temperature/ applied electrical current this may be achieved as follows:
Alternatively if we need a valve which closes with an increase in temperature / applied electrical current this could simply be achieved by expanding inside the duct to constrict/block the flow of air.
Dielectric Elastomers
DE valves could be controlled by a microprocessor with inputs from temperature sensors at critical points in the system as originally proposed. This would require an electrical connection between the microprocessor and each of the DE valves which would add to the complexity of the system but could still be possible.
Shape Memory Alloys
SMA valves could be controlled by directly responding to the temperature of their locality. In this case maybe the SMA valves would work like logic gates in an electric circuit, taking air intake temperature as the inputs and the critical cold store temperature as the desired output.
Although this may be extremely complex to design and build initially but such a system would theoretically be completely electric free.
Alternatively the SMA valves could be controlled by electric heating elements via microprocessor system as explained above.
In any case the advantage of using smart materials instead of conventional valves (e.g solenoid) would be:- No mechanically moving parts so could be more robust, reliable and longer lasting
- Could be made in much smaller dimensions.
- Could easily be made to any customised size as required, whereas conventional valves are usually only manufactured to standard sizes.
- Could be much cheaper
Tancio suggested we could somehow incorporate smart materials into our control system for the adsorption refrigeration cycle.
Smart materials have special properties which cause them to undergo some specific change when induced to external stimuli.
Referring back to the idea of the termite ventilation system (where natural buoyancy forces are cleverly manipulated by dynamically opening/closing ducts to control the flow of air through the system at the correct rates to maintain the required temperatures.
Designing such a system would firstly require a thorough understanding of the thermodynamic principles of this complex system which may require computer modelling.
In any case, we would require some form of actuator to open and close the ducts. Since it may be difficult to hire an army of termites to do this for us, in our case we may be able to use smart materials our actuators.
Dielectric Elastomers (DE) and Shape Memory Alloys (SMA) are particular smart materials which may be useful for this application as they expand when induced to an electric current and heat respectively. They could therefore be used as air flow control valves by using their expansion process to constrict or open ducts as required.
If we need a valve which opens with an increase in temperature/ applied electrical current this may be achieved as follows:
Alternatively if we need a valve which closes with an increase in temperature / applied electrical current this could simply be achieved by expanding inside the duct to constrict/block the flow of air.
Dielectric Elastomers
DE valves could be controlled by a microprocessor with inputs from temperature sensors at critical points in the system as originally proposed. This would require an electrical connection between the microprocessor and each of the DE valves which would add to the complexity of the system but could still be possible.
Shape Memory Alloys
SMA valves could be controlled by directly responding to the temperature of their locality. In this case maybe the SMA valves would work like logic gates in an electric circuit, taking air intake temperature as the inputs and the critical cold store temperature as the desired output.
Although this may be extremely complex to design and build initially but such a system would theoretically be completely electric free.
Alternatively the SMA valves could be controlled by electric heating elements via microprocessor system as explained above.
In any case the advantage of using smart materials instead of conventional valves (e.g solenoid) would be:- No mechanically moving parts so could be more robust, reliable and longer lasting
- Could be made in much smaller dimensions.
- Could easily be made to any customised size as required, whereas conventional valves are usually only manufactured to standard sizes.
- Could be much cheaper
Tancio suggested we could somehow incorporate smart materials into our control system for the adsorption refrigeration cycle.
Smart materials have special properties which cause them to undergo some specific change when induced to external stimuli.
Referring back to the idea of the termite ventilation system (where natural buoyancy forces are cleverly manipulated by dynamically opening/closing ducts to control the flow of air through the system at the correct rates to maintain the required temperatures.
Designing such a system would firstly require a thorough understanding of the thermodynamic principles of this complex system which may require computer modelling.
In any case, we would require some form of actuator to open and close the ducts. Since it may be difficult to hire an army of termites to do this for us, in our case we may be able to use smart materials our actuators.
Dielectric Elastomers (DE) and Shape Memory Alloys (SMA) are particular smart materials which may be useful for this application as they expand when induced to an electric current and heat respectively. They could therefore be used as air flow control valves by using their expansion process to constrict or open ducts as required.
If we need a valve which opens with an increase in temperature/ applied electrical current this may be achieved as follows:
Alternatively if we need a valve which closes with an increase in temperature / applied electrical current this could simply be achieved by expanding inside the duct to constrict/block the flow of air.
Dielectric Elastomers
DE valves could be controlled by a microprocessor with inputs from temperature sensors at critical points in the system as originally proposed. This would require an electrical connection between the microprocessor and each of the DE valves which would add to the complexity of the system but could still be possible.
Shape Memory Alloys
SMA valves could be controlled by directly responding to the temperature of their locality. In this case maybe the SMA valves would work like logic gates in an electric circuit, taking air intake temperature as the inputs and the critical cold store temperature as the desired output.
Although this may be extremely complex to design and build initially but such a system would theoretically be completely electric free.
Alternatively the SMA valves could be controlled by electric heating elements via microprocessor system as explained above.
In any case the advantage of using smart materials instead of conventional valves (e.g solenoid) would be:- No mechanically moving parts so could be more robust, reliable and longer lasting
- Could be made in much smaller dimensions.
- Could easily be made to any customised size as required, whereas conventional valves are usually only manufactured to standard sizes.
- Could be much cheaper
Tancio suggested we could somehow incorporate smart materials into our control system for the adsorption refrigeration cycle.
Smart materials have special properties which cause them to undergo some specific change when induced to external stimuli.
Referring back to the idea of the termite ventilation system (where natural buoyancy forces are cleverly manipulated by dynamically opening/closing ducts to control the flow of air through the system at the correct rates to maintain the required temperatures.
Designing such a system would firstly require a thorough understanding of the thermodynamic principles of this complex system which may require computer modelling.
In any case, we would require some form of actuator to open and close the ducts. Since it may be difficult to hire an army of termites to do this for us, in our case we may be able to use smart materials our actuators.
Dielectric Elastomers (DE) and Shape Memory Alloys (SMA) are particular smart materials which may be useful for this application as they expand when induced to an electric current and heat respectively. They could therefore be used as air flow control valves by using their expansion process to constrict or open ducts as required.
If we need a valve which opens with an increase in temperature/ applied electrical current this may be achieved as follows:
Alternatively if we need a valve which closes with an increase in temperature / applied electrical current this could simply be achieved by expanding inside the duct to constrict/block the flow of air.
Dielectric Elastomers
DE valves could be controlled by a microprocessor with inputs from temperature sensors at critical points in the system as originally proposed. This would require an electrical connection between the microprocessor and each of the DE valves which would add to the complexity of the system but could still be possible.
Shape Memory Alloys
SMA valves could be controlled by directly responding to the temperature of their locality. In this case maybe the SMA valves would work like logic gates in an electric circuit, taking air intake temperature as the inputs and the critical cold store temperature as the desired output.
Although this may be extremely complex to design and build initially but such a system would theoretically be completely electric free.
Alternatively the SMA valves could be controlled by electric heating elements via microprocessor system as explained above.
In any case the advantage of using smart materials instead of conventional valves (e.g solenoid) would be:- No mechanically moving parts so could be more robust, reliable and longer lasting
- Could be made in much smaller dimensions.
- Could easily be made to any customised size as required, whereas conventional valves are usually only manufactured to standard sizes.
- Could be much cheaper
Smart materials have special properties which cause them to undergo some specific change when induced to external stimuli.
Referring back to the idea of the termite ventilation system (where natural buoyancy forces are cleverly manipulated by dynamically opening/closing ducts to control the flow of air through the system at the correct rates to maintain the required temperatures.
Designing such a system would firstly require a thorough understanding of the thermodynamic principles of this complex system which may require computer modelling.
In any case, we would require some form of actuator to open and close the ducts. Since it may be difficult to hire an army of termites to do this for us, in our case we may be able to use smart materials our actuators.
Dielectric Elastomers (DE) and Shape Memory Alloys (SMA) are particular smart materials which may be useful for this application as they expand when induced to an electric current and heat respectively. They could therefore be used as air flow control valves by using their expansion process to constrict or open ducts as required.
If we need a valve which opens with an increase in temperature/ applied electrical current this may be achieved as follows:
Alternatively if we need a valve which closes with an increase in temperature / applied electrical current this could simply be achieved by expanding inside the duct to constrict/block the flow of air.
Dielectric Elastomers
DE valves could be controlled by a microprocessor with inputs from temperature sensors at critical points in the system as originally proposed. This would require an electrical connection between the microprocessor and each of the DE valves which would add to the complexity of the system but could still be possible.Shape Memory Alloys
SMA valves could be controlled by directly responding to the temperature of their locality. In this case maybe the SMA valves would work like logic gates in an electric circuit, taking air intake temperature as the inputs and the critical cold store temperature as the desired output.Although this may be extremely complex to design and build initially but such a system would theoretically be completely electric free.
Alternatively the SMA valves could be controlled by electric heating elements via microprocessor system as explained above.
In any case the advantage of using smart materials instead of conventional valves (e.g solenoid) would be:
- No mechanically moving parts so could be more robust, reliable and longer lasting
- Could be made in much smaller dimensions.
- Could easily be made to any customised size as required, whereas conventional valves are usually only manufactured to standard sizes.
- Could be much cheaper
Problems with pressurised ammonia
Hit a brick wall with the building of the absorption refrigeration (Einstein’s fridge and solar ice maker) prototypes.
Having acquired most of the materials and completed construction of the condenser, heat exchanger and solar concentrator I have realised we are unable to fill the system with pressurised ammonia unless we comply with the never ending list of regulations for pressure vessels and even more regulations for using ammonia.
Critically, all welding must be done by a coded welder and the completed system must be inspected and underwritten by an insurance company. This means it will be far more expensive and time consuming than originally expected.
Furthermore I have realised that using pressurised ammonia means all the pipes and fittings have to be of very high specifications to comply with the safety regulations which also adds to the cost. For example for the solar ice maker the two valves alone costed £69 , the two end caps for the generator costed £75 as they had to be made of steel to resist ammonia attack and withstand the pressure of 200psi (actually rated to 1000psi for steam).
Even if we were to complete the construction of this prototype, how sustainable would this solution be for implementation in rural locations in developing countries? Where would we find a coded welder and all the high spec parts locally? Even so, would it be financially viable to construct and maintain in the long term?
On the other hand the proposed adsorption system would operate on air/ water vapour at atmospheric pressure. Therefore it could be made with much cheaper, easier and safer to construct and maintain locally.
Therefore I am considering discontinuing the construction of absorption refrigeration system prototypes to concentrate future work on developing the proposed adsorption system.
Sunday 22 August 2010
Two stage – adsorption / evaporative cooling
Looking at the wet bulb temperatures for Gambia we can see that there is little cooling in the rainy season (June to Oct) when the humidity is high. So I was thinking if there was some way of reducing humidity so we could deliver effective evaporative cooling all year round.
It turns out there are certain (hygroscopic) materials which have an affinity to draw moisture from air (adsorption). So if we were to pass this a hygroscopic material (desiccant) it would come out drier so would give a lower wet bulb temperature (more effective evaporative cooling).This process is called adsorptive cooling and has already been used for storage of vaccines and food in developing countries (reference).
But maybe we could improvement on this by taking the cold air coming out of the evaporative cooler, drying it again, and the cooling it even further by sending it though another stage of evaporative cooling.
I have shown this proposed process on the psychometric chart below, using the example of typical ambient air during the day in the rainy season in Gambia (30oC , 80% relative humidity).
Figure 1: Comparison of direct evaporative cooling to proposed two stage adsorption cooling process
Regeneration methods
Now in order for this process to be continuous it would require some method of removing moisture (desorption) from the desiccant.If the desiccant is liquid (eg. Saturated Calcium Chloride solution), the diluted solution (after water is adsorbed from wet air) is usually dried by pumping it through a heat exchanger causing the water to boil off, thus re-concentrating it and so allowing it to adsorb more moisture from incoming air, as shown in the diagram below.
Figure 2: Conventional method of dehumidifying air by adsorption and regeneration of liquid desiccant
However, pumping the solution through a heat exchanger would require a significant electrical power input. Maybe we could use the following alternative process:
Figure 3: Proposed method of dehumidifying air and regenerating liquid desiccant without requiring any electrical inout for pumping.
A drying chamber is filled with the saturated solution; with excess solid precipitate of the desiccant of added.
Air is passed through the solution causing it to dry out, giving up its moisture to the desiccant. This causes the solution to dilute and therefore its volume increases.
As the volume of the solution increases, it overflows the drying chamber and is is forced by constriction into the heat exchanger. The heat exchanger is simply a pipe with solar radiation concentrated on it by a parabolic solar trough.
This solar heating causes the extra water to evaporate off maintaining the saturation of the solution without the use of any electrical power requirement for pumping.
If the desiccant is a solid (eg. Charcoal, silica gel, activated alumina, lithium chloride salt or synthetic polymers) a usually this system is regenerated (re-dried) using a rotary desiccant wheel.
Some air is superheated, by solar concentration/ collection and blown and also passed though the wheel for desorption.
The rotation of the wheel allows for the continuous and simultaneous adsorption and desorption.
However the rotation of the wheel would also required a significant electrical power input. Maybe we could achieve the same end without even moving the porous desiccant material, since if part of it is maintained dry by blowing hot air though and the other is being wetted by adsorption from incoming wet air, this would set up a water concentration gradient from the wet to the dry part, across which the water could flow by osmosis, since the material itself would be a semi-permeable membrane due to its porosity.
Of course this process would not be as fast as using rotating wheel, but maybe if we chose an optimum porous material and desiccant and possible some other substance to catalyse the diffusion process this process could become feasible.
Overall process
I have set out a flow diagram to explain the overall process (in this case assuming the use of a solid porous desiccant:Figure 4: Flow diagram to explain the proposed process two stage adsorption / evaporative cooling
As suggested in this diagram, we could recycle part of the desorbed from the desiccant by the hot air in the regeneration process by passing though a condenser cooled by ambient air. This recycled water could be used to feed the evaporative cooling process. Calculations are required to verify the feasibility of obtaining all the water needed for this process by this method.
Supplementary water and heat backup
If more water is required this could be provided by the intermittent addition of water from a nearby well as this will definitely be available in any location where there is vegetable farming activity.If a groundwater supply is provided this could also function as am additional cold sink to cool the condenser and also the incoming ambient air as this water is usually significantly colder than the ambient air during the day as there is a thermal lag due to the time required for the sun to heat the large underground reservoirs.
Similarly if our calculations were to suggest there maybe times when there is insufficient supply from the solar concentrator, this could be supplemented by burning firewood, cow dung, waste agricultural material, charcoal or cooking gas.
This could require a display and alarm on the control system which would alert the users when water / heat levels are critically low so they could the required actions accordingly.
Two level cooling
Also, as shown in the flow chart, it should be possible to provide to levels of cold storage using this method. The first level would be moderately cool, supplied by air from the first cycle of evaporative cooling. The second level would be further chilled using air from the second stage of cooling.This would allow the system to be used for simultaneous storage of a greater variety of fruit and vegetables (and maybe even dairy products) all within their optimum conditions.
Termite inspired natural ventilation
The entire process would required significant flow rates of air, through the desiccant dehumidifiers, the evaporative coolers, over and through the condenser, and for the circulation of air within the cold stores which would require a significant electrical power input if driven by fans.So maybe we could cleverly make use of natural buoyancy forces (hot air rises, cold air sinks) to drive the flow of air instead, just as termites do to control the temperature of their mounds.
Figure 5: Termites’ use natural convection to ventilate their mounds
Within these mounds the termites control the exact temperature of a certain section at the optimum level for farming fungus for their food, and also another separate section is maintained at the optimum temperature for the Queen termite! This control is maintained perfectly throughout the year despite the dramatically variable day/night, daily and seasonal temperature and humidity environmental conditions they are situated in.
The process is controlled by an elaborate network of channels which draw cold air in from the bottom and ventilate hot air out though the top. The termites open and close thousands of tiny ducts all over the mound (using mud and their saliva) to control the conditions in the mound to their exact requirements.
Now in our case we could use a micro-processor system taking inputs from humidity and temperature sensors at the critical points in the system and accordingly opening/closing valves (as output) to control the flow of air though a network of pipes / ducts to ultimately control the temperatures in the cold stores.
Monday 16 August 2010
Wet bulb temperatures Gambia
The “ambient” values were obtained from looking up data for the max average temperature found here.
The wet bulb temperature was calculated from the respective ambient temperatures and relative humanities (again from here - lookup “pm”) using this applet (setting pressure to 760mmHg = 1atm). This could also be found by using a psychometric chart but I couldn't be bothered.
Overlaid are the upper limit for the optimum cold storage temperatures for fruit and vegetables which are commonly grown and consumed (or wasted) in Gambia.
This may explain the excellent results recorded from the experiment conducted at the University of The Gambia in January 2009 where it was shown that the shelf life of tomatoes could be significantly extended in the Desert Fridge (see report here).
However it also reveal that the Desert Fridge would not be adequate for storage of most fuit and vegetables throughout the year. Conveniently though, the greatest demand for storage in Gambia we have observed to be is in the dry season (oct/nov to may/jun) and that is of tomatoes.
Nevertheless this means we need to develop an improved solution to meed these requirements.
Sunday 15 August 2010
Inner pot DF: Steel vs Aluminium (more new results)
Inner pot DF: Steel vs Aluminium (more new results)
The results of the first run of the experiment to compare the effectiveness of Steel vs Aluminium as the material for the inner pot material were very strange so I decided to re-run it and a summary of the results is given below (complete data here and notes) :
Run 1: 16/7/10 See previous post
See previous post

Run 2: 22/7/10  To reduce heat transfer from oven straight through to inner pot, the top surface of both the Desert Fridges were completely insulated with a sheet of polystyrene.
To reduce heat transfer from oven straight through to inner pot, the top surface of both the Desert Fridges were completely insulated with a sheet of polystyrene.
Furthermore, efforts were made to ensure the thermocouples remained in the exact positions they were supposed to be kept in using blu- tack and selotape to hold them in place.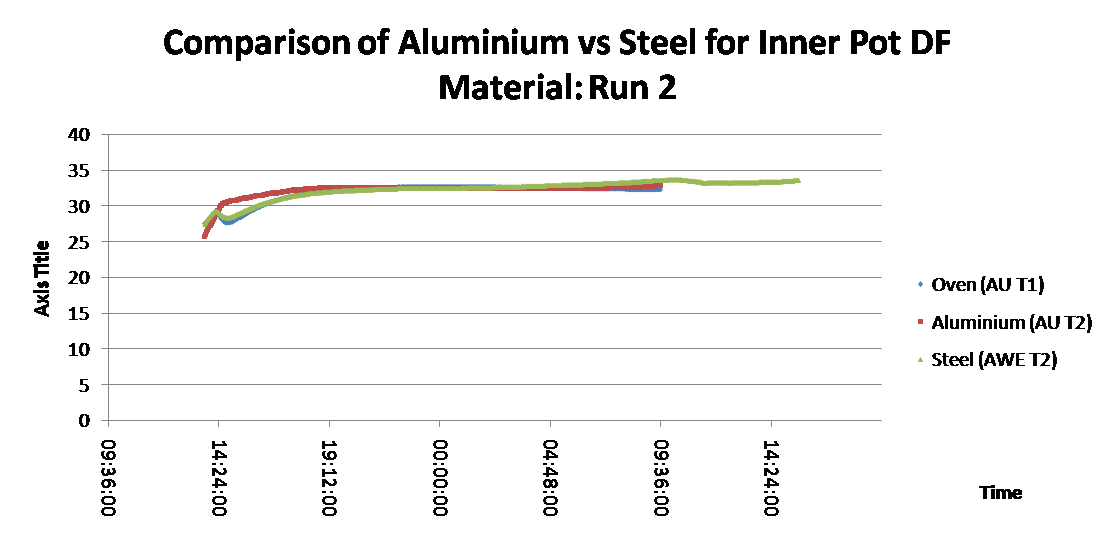
Initially there was some small deviation of the aluminium above the oven temperature (reason unknown) while the system warmed up. Once equilibrated, both the steel and aluminium showed virtually zero difference to the surrounding oven temperature. This may have been because the insulating layer added would have prevented evaporation of water from the exposed sand layer at the top surface which would reduce the cooling effect.
Run 3: 26/7/10  This time, to allow evaporation of water from the exposed sand while reducing heat from the surroundings to the inner pot; the insulting layer of polystyrenes was cut such that it only covered the top of the inner pot while leaving the sand section exposed.
This time, to allow evaporation of water from the exposed sand while reducing heat from the surroundings to the inner pot; the insulting layer of polystyrenes was cut such that it only covered the top of the inner pot while leaving the sand section exposed.
This gave the best results, i.e the largest sustained temperature difference (approx 5oc) between the inner pot (both and surrounding oven temperature. The steel pot was negligibly (less than 0.1oC) cooler than aluminium, but this is so small it could have been due to the slight variation in temperature due to its position within the oven or the slight variations in the outer pot (clay) and/or sand layer material properties, rather than due to inner pot material itself.
However the performance of both DFs was still much worse than that recorded in previous similar experiments where a sustained temperature difference of 10oC was maintained.
Conclusions
This series of experiments suggests that there is no significant difference in performance of the Desert Fridge between using aluminium or steel as the inner pit material.
Furthermore, we can conclude that the only significant heat transfer process is though the top surface of the Desert Fridge. If this is true it would completely contradict the 1-D theoretical model of the DF we have been developing which assumes the heat transfer in this direction is insignificant compared to that through the walls of the outer pot.
Porosity of outer clay pot
However these results may be because in this specific experiment the outer pots were not significantly porous, as if this were the case then there would be insignificant diffusion of water and resultant heat transfer process in this direction.
The porosity of the terracotta outer pots were tested for by spraying the surface with water and a positive results was observed as the water was quickly absorbed. Furthermore, when the pots were submerged in water to be saturated for the experiments air bubble evolving from the surface of the pots were observed which also suggested they were porous.
However on further consideration, these tests only proved the pots were porous but even if there were few/ no interconnected pathways of theses pores from the inner to the outer surface, then the hydraulic conductivity and the resultant heat transfer process could still have been insignificant.
Here is a method of accurately determining the hydraulic conductivity of terracotta pots. However this is very time consuming. In any case is very difficult to accurately reproduce terracotta pots with the same porosity as the it is affeceted by so many variables including: clay type, firing temperature, molding method, additives and water content etc . Even if we are able to reproduce samples in lab conditions will this really be practical for implementation? Therefore should we consider discontinuing investigations using terracotta pots and concentrate on other more reliable materials?...
Inner pot DF: Steel vs Aluminium (more new results)
The results of the first run of the experiment to compare the effectiveness of Steel vs Aluminium as the material for the inner pot material were very strange so I decided to re-run it and a summary of the results is given below (complete data here and notes) :
Run 1: 16/7/10 See previous post
See previous post
Run 2: 22/7/10  To reduce heat transfer from oven straight through to inner pot, the top surface of both the Desert Fridges were completely insulated with a sheet of polystyrene.
To reduce heat transfer from oven straight through to inner pot, the top surface of both the Desert Fridges were completely insulated with a sheet of polystyrene.
Furthermore, efforts were made to ensure the thermocouples remained in the exact positions they were supposed to be kept in using blu- tack and selotape to hold them in place.Initially there was some small deviation of the aluminium above the oven temperature (reason unknown) while the system warmed up. Once equilibrated, both the steel and aluminium showed virtually zero difference to the surrounding oven temperature. This may have been because the insulating layer added would have prevented evaporation of water from the exposed sand layer at the top surface which would reduce the cooling effect.
Run 3: 26/7/10  This time, to allow evaporation of water from the exposed sand while reducing heat from the surroundings to the inner pot; the insulting layer of polystyrenes was cut such that it only covered the top of the inner pot while leaving the sand section exposed.
This time, to allow evaporation of water from the exposed sand while reducing heat from the surroundings to the inner pot; the insulting layer of polystyrenes was cut such that it only covered the top of the inner pot while leaving the sand section exposed.
This gave the best results, i.e the largest sustained temperature difference (approx 5oc) between the inner pot (both and surrounding oven temperature. The steel pot was negligibly (less than 0.1oC) cooler than aluminium, but this is so small it could have been due to the slight variation in temperature due to its position within the oven or the slight variations in the outer pot (clay) and/or sand layer material properties, rather than due to inner pot material itself.However the performance of both DFs was still much worse than that recorded in previous similar experiments where a sustained temperature difference of 10oC was maintained.
Conclusions
This series of experiments suggests that there is no significant difference in performance of the Desert Fridge between using aluminium or steel as the inner pit material.Furthermore, we can conclude that the only significant heat transfer process is though the top surface of the Desert Fridge. If this is true it would completely contradict the 1-D theoretical model of the DF we have been developing which assumes the heat transfer in this direction is insignificant compared to that through the walls of the outer pot.
Porosity of outer clay pot
However these results may be because in this specific experiment the outer pots were not significantly porous, as if this were the case then there would be insignificant diffusion of water and resultant heat transfer process in this direction.The porosity of the terracotta outer pots were tested for by spraying the surface with water and a positive results was observed as the water was quickly absorbed. Furthermore, when the pots were submerged in water to be saturated for the experiments air bubble evolving from the surface of the pots were observed which also suggested they were porous.
However on further consideration, these tests only proved the pots were porous but even if there were few/ no interconnected pathways of theses pores from the inner to the outer surface, then the hydraulic conductivity and the resultant heat transfer process could still have been insignificant.
Here is a method of accurately determining the hydraulic conductivity of terracotta pots. However this is very time consuming. In any case is very difficult to accurately reproduce terracotta pots with the same porosity as the it is affeceted by so many variables including: clay type, firing temperature, molding method, additives and water content etc . Even if we are able to reproduce samples in lab conditions will this really be practical for implementation? Therefore should we consider discontinuing investigations using terracotta pots and concentrate on other more reliable materials?...
Control system for prototypes
Flow rate
In order to design the electrical circuit we will need to know the required power supply to the pump, which will be determined by the flowrate required from the pump which will be determined by:
1) temperature of the cold store should be maintained :lets say 10oC as a target to start with)
2) the heat exchangers: as per air-side HE, acquired from back of fridge in skip with configuration as shown below (still need to measure pipe bore and thickness - material = steel):
This heat exchanger has 100 fins (50 front - 50 back) over its surface. However may be too big to fit it all in our prototype cold store (a small fridge) and so we can cut it down if required.
(For the cold thermal fluid store side HE, im not sure what we are going to use - because this needs to be under pressure and also contain a significant mass of fluid as it is the evaporator of the absorption system. ... do you even need an effective heat exchanger design as there would the same amount of heat be extracted from the surroundings regardless of design .s For normal heat exchange processes, for a given temperature difference, increasing the heat exchange surface area and conductivity will increase rate of heat transfer.... but in our case there is no temperature differential driving the process it is the vaporisation of the refrigerant which would draw the same amount of heat (depending on mass vaporised) regardless of these heat exchanger parameters.... hrreer maybe the heat extracted from the surrounding of the total heat removed by evaporation (remaining part drawn from air/gas side inside evaporator?) would be proportional to the heat exchanger effectiveness... therefor it would be critical....?)
3) Coolant :lets use water - or salt water as this has a lower freezing temp
4) volume of air to be cooled: 40 litres (waeco fridge)
Electrical
Have acquired a car batter (12v, 380 A) and a car windscreen pump (12v) from a scrap yard and some crocodile clips, 2 variable resistors (0-1K and 0- 4.7K ohms) and a PTC thermistor (1.19K ohm @ 100oC; 15K ohm @ 25oC; 45.13 ohm@ 0oC) from Maplins.
Was thinking the circuit would look something like this:
So when the temperature in the cold store is too high the resistance of the thermistor would be high, so more current would be drawn through the pump arm of the circuit, driving the pump faster and thus cooling the cold store and visa-versa.
The variable resistor would be used to set the constant temperature (lower resistance = more power to pump = cooler)
The second constant resistance component would be used ensure the overall resistance of the circuit is adequate.
Now since I did not have enough information about the components exact specifications I tried to find a working solution by playing with different configurations. Whatever I tried, the pump would wither run at full power or zero. I eventually gave up with this method when I fried my other thermistor (NTC one) and the variable resistors started glowing red!
Will need to work on this...
Friday 13 August 2010
Wednesday 11 August 2010
Thermoelectric Peltier effect and Phase Change Materials
Monday 9 August 2010
Solar cold store with control system only
1) Combined sweat cooling and semi-continuous solar ice maker powered control system:
For this method first we would encapsulate the entire chamber it in a moisture containing layer, supplied with water by a drip line fed by a raised reservoir.
This would reduce to the load on the active cooling cycle as it would reduce the surrounding temperature to the wet bulb temperature of the ambient surrounding (which can be as much as 10-15oC cooler).
Now to reduce the temperature further (as much as is required), the modified version of the solar absorption ice maker system which allows for semi-continuous operation (continuous during day - at variable rates depending on climate) using the same water reservoir feeding the sweat cooling system
2) Einstein’s fridge powered control system :
In either case, the evaporators would not cool the cold storage directly but would cool in intermediate low temperature thermal storage fluid (eg water/ice). The design of the evaporator tanks would be optimised to facilitate efficient heat transfer.
The cooling delivered by this system as it is would be variable as the power delivered from the sun will always be variable which would not ideal as for proper cold storage a constant temperature should be maintained.
This could be possible by controlling the rate of heat transfer (using an electrconic control system run on a car battery) from the thermal storage fluid to the air in the cold storage chamber by pumping (pump also run on car battery) a fluid a controlled rate through a heat exchanger inside the thermal fluid and in turn through a heat exchanger with the cold storage chamber air (which will have fins to increase surface area so a fan would not be required (thank you Sampal for the fins idea :) )
So when there is surplus heat from the sun it would deliver extra cooling to the thermal fluid so the pump would run slower and when there is less cooling delivered by the evaporators, the pump would run faster to compensate. Therefore we could maintain any constant specified temperature inside the cold store with only solar power...
Now lets build this thing :)
Sunday 8 August 2010
Sweat Cooling / Ammonia Absorption / Gas Hybrid Cold Store
However neither of the systems we have investigated so far are able to meet these specifications.
Simple evaporation of water (as used in the original Desert Fridge ) can somewhat extend the life of some products (such as tomatoes because they happen to have an optimum storage temperature of 17-21oC) but it is limited to the wet bulb temperature so will never be able to store most other vegetables for any significant length of time. Also it requires a hot AND dry climate to operate which is fine in some places at certain time for example in Gambia it works well in the dry season but not the wet season.
The solar ammonia absorption ice maker could get the temperature down to freezing (or even below i think) which would be enough to store anything but the cooling cycle would only operate at night. Even if we used the suggested modification to allow continuous cooling this would only be “continuous” during the day (since the heat cycle would not run at night) and again this would require a condusive climate for evaporation of water which can not be relied on.
Einstein’s fridge could work continuously and deliver the required cooling but required burning gas a a heat source which would be too expensive to run in the long term. If we used solar power as the heat source it would not be continuous and reliable.
Using a thermal storage fluid could help by storing surplus heat, by storing the thermal fluid in an insulated container, and returning it, by pumping it to the generator when required.
Also we must consider the use of a fan (run on a car battery) inside a room of this capacity to increase the rate of heat transfer from the immediately cooled (by conduction) section to the remaining air (by convection). Now the speed of this fan could also be controlled to facilitate continuous constant cooling - when the sun is delivering surplus power, more/cooler ice (or whatever coolant) is produced so the fan could run on lower power and visa-versa.
Another way of tackling the problem of the unreliability of solar power is to use kerosene or any other locally available cooking gas as a backup heat source.
Maybe the best way to proceed is to design a hybrid system combining the best of all these ideas. For example lets consider something like this....
This would require an electronic control system (again run on the car battery) would be required to control any combination of (whichever works out best) the gas burner, the fan and the thermal fluid, facilitating the primary solar power, to maintain the temperature in the cold store constantly at the required temperature 24/7! Some may say it defeats the object of the original simple Desert Fridge, but im sure with a bit (actually a lot) of clever engineering we could adapt this process to make it feasible for application in developing countries.
